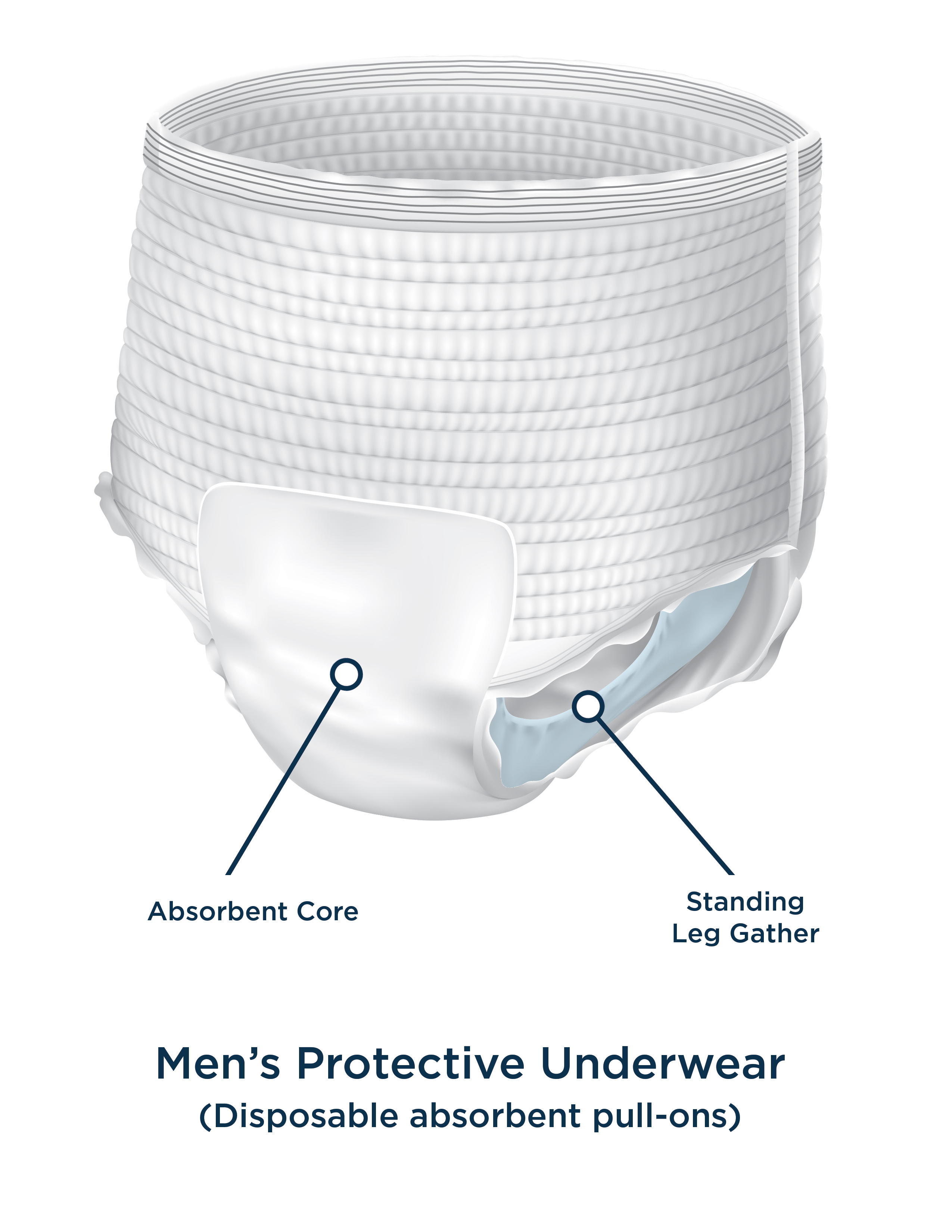
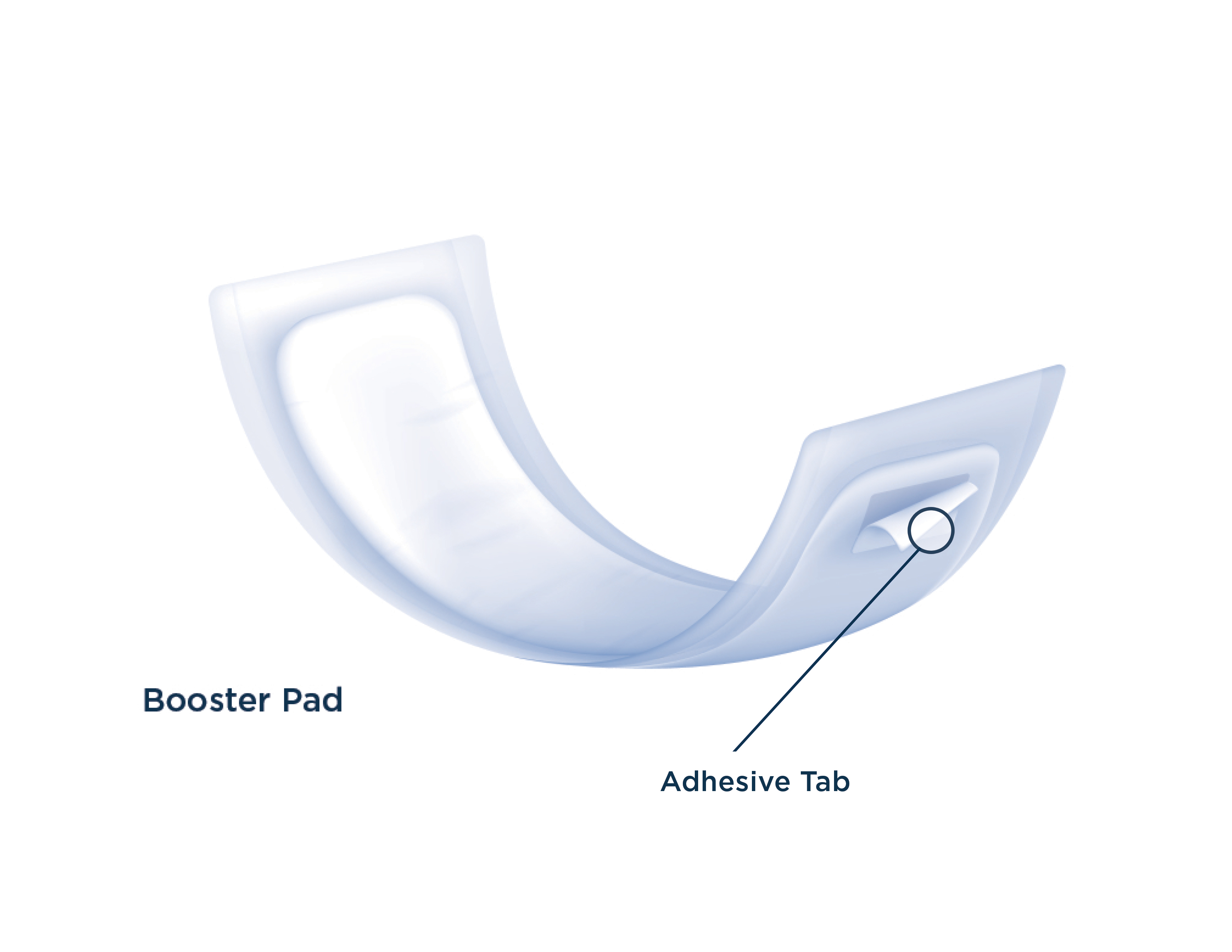
INTRODUCTION
Despite recent advances in multiple areas of continence management including pharmacotherapy, surgery, physiotherapy, and neuromodulation, evidence suggests that use of incontinence products remains the most prevalent strategy among adults with urinary or fecal incontinence. To address this gap, the WOCN Society developed an evidence- and consensus-based algorithm for selection, use, and evaluation of body worn absorbent products for the management of individuals with urinary and/or fecal incontinence. This algorithm will help to fill the gap in resources available for first-line and WOC specialty practice nurses guiding optimal use of these products.
To learn more about the algorithm’s development, we invite you to read the accompanying article, “Assessment, Selection, Use, and Evaluation of Body Worn Absorbent Products for Adults With Incontinence: A WOCN Society Consensus Conference”, published in Journal of Wound, Ostomy and Continence Nursing: May/June 2018 - Volume 45 - Issue 3 - p 243–264.